General Chemistry 2: Rates of Reaction
Consider a solution of 0.10 M H in 0.10 M thioacetimide at 25 C
rate = k[H][CHCSNH]
How are the rate and the rate constant (k) effected when water is added to the solution? When the reaction is heated to 75C ? When NaOH is added?
The initial concentration of hydroiodic acid is 100 mmol/L but after 500 s it is 17 mmol/L. What is the rate? (note the products of this reaction are hydrogen and iodine gas)
The following initial rate data was collected for the reaction, A + 2B products

B. Find the rate constant
The following initial rate data was collected for the reaction, 2 NO + O 2NO

[NO][O]
The following initial rate data was collected for the reaction, CHBr + OH CHOH + Br
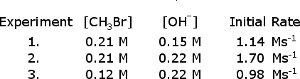
B. What is the rate constant?
The following initial rate data was collected for the reaction, A + B + C products

What are the reaction orders with respect to the reactants?
When cyclopropane is heated to 500 C it changes to propene. The following data was obtained from experiment:

Confirm that the reaction is 1st order and calculate the rate constant.
An isotope of phosphorous, P, is radioactive and undergoes beta decay with a half life of 14.3 days. How long would it take for 99% of a sample of this isotope to decay?
A products
t = 180s
A. What % of A is left unreacted at 900 s?
B. What is the rate at [A] = 0.50 M?
The half life of uranium-238 is 4.51 x 10 years. What is the rate constant? How much uranium-238 is left after 4 half lives if we start with 64 mg?
A first order reaction has a half life of 20.0 min.
A. Calculate the rate constant for this reaction
B. How much time is required for this reaction to be 75% complete?
For a given reaction, the reaction rate exactly doubles when the temperature is raised from 293 K to 304 K. Calculate the activation energy.
Below is a proposed mechanism for a reaction, with k2 > k1. What is the rate law?
Below is a proposed mechanism for a reaction, with k1 > k2. What is the rate law?
Below is a proposed mechanism for a reaction, with k1 > k2. What is the rate law?
For the mechanism proposed below, determine the overall rate law for k < k, k > k
For the mechanism proposed below, use the quasi (pseudo) steady state approximation to determine the overall rate law assuming the pseudo steady state.
Use the pseudo steady state hypothesis to derive the rate law for the rate of production of P