Rates of Reaction Problem
Consider a solution of 0.10 M H in 0.10 M thioacetimide at 25 C
rate = k[H][CHCSNH]
How are the rate and the rate constant (k) effected when water is added to the solution? When the reaction is heated to 75C ? When NaOH is added?
Posted by Andrew Gomez 2 years ago
Related Problems
The initial concentration of hydroiodic acid is 100 mmol/L but after 500 s it is 17 mmol/L. What is the rate? (note the products of this reaction are hydrogen and iodine gas)
The following initial rate data was collected for the reaction, A + 2B products

B. Find the rate constant
The following initial rate data was collected for the reaction, 2 NO + O 2NO

[NO][O]
The following initial rate data was collected for the reaction, CHBr + OH CHOH + Br
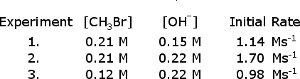
B. What is the rate constant?