General Chemistry 1: Ideal Gas Law
The density of sea water is 1.03 g/mL. What mass of seawater would fill a vessel to a volume of 255 mL?
An ocean dwelling dinosaur has an estimated body volume of 1.38 x 10 cm. The animals's live mass was estimated at 1.24 x 10 g. What is its density?
A student collected a gas in an apparatus connected to an open end manometer. The difference in heights of mercury in the two columns was 102 mm and the atmospheric pressure was measured to be 756 mmHg. What was the pressure of the gas in the apparatus in atm?
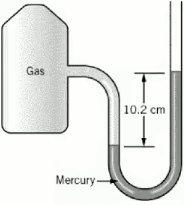
A sample of nitrogen has a volume of 883 mL and a pressure of 741 torr. What pressure will change the volume to 655 mL at the same temperature?
What will be the final pressure of a sample of nitrogen with a volume of 955 mL at 745 torr and 25 C if it is heated to 62 C and given a final volume of 1155 mL?
How many liters of hydrogen, measured at STP, are needed to combine exactly with 1.50 L of nitrogen, also at STP, to form ammonia?
n + 3 H 2 NH
How many grams of Ar are in a 12.0 L cylinder of argon at a pressure of 57.8 atm and a temperature of C ?
The label on a cylinder of a noble gas became illegible, so a student allowed some of the gas to flow into a gas bulb with a volume of 0.3000 L until the pressure was 0.901 atm. The sample then weighed 1.45 g and its temperature was 27.0 C. What is the molar mass of the gas? Which noble gas is it?
One procedure used to separate the isotopes of uranium to obtain material to construct a nuclear weapon employs uranium compound with the formula UF . The compound boils at about 56 C, so at 100 C it is a gas. What is the density of 1.00 mol of UF at 100 C if the pressure of the gas is 0.974 atm. (MM of U = 352.0 g/mol)
Suppose a 1.25 g sample of CaCO is decomposed by heating. How many milliliters of CO gas will be evolved if it will be measured at 0.974 atm and 25 C (MM of CaCO = 100.05 g/mol)
CaCO (s) CO (g) + CaO (s)
A sample of gas has a pressure of 1.5 atm and a volume of 500.0 mL. What would be the pressure if the volume were compressed to 325.0 mL at a constant temperature?
Find the molar mass of 15.0 g of a gas in a 2.0 L container at 291 K and 1.3 atm.
Calculate the density (g/L) of methane ( CH ) at 1.0 atm and 278 K.
Mg(s) + 2 HCl(aq) MgCl(aq) + H(g)
50.0 mL of 3.0 M HCl reacts with excess Mg in a closed 4.0 L container at 295 K. What is the pressure from the hydrogen gas that is produced?
A sample of oxygen is collected over water at 20 C and a pressure of 738 torr. What is the partial pressure of oxygen in mmHg??
Rank the following gasses in order of increasing rates of effusion:
O
Ar
CO
N
He
SO