Molar Mass of an Unknown Gas
Find the molar mass of 15.0 g of a gas in a 2.0 L container at 291 K and 1.3 atm.
Posted by Katie Adams 2 years ago
Related Problems
A student collected a gas in an apparatus connected to an open end manometer. The difference in heights of mercury in the two columns was 102 mm and the atmospheric pressure was measured to be 756 mmHg. What was the pressure of the gas in the apparatus in atm?
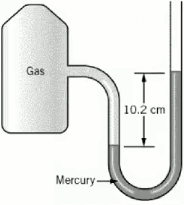
A sample of nitrogen has a volume of 883 mL and a pressure of 741 torr. What pressure will change the volume to 655 mL at the same temperature?
Calculate the density (g/L) of methane ( CH ) at 1.0 atm and 278 K.
Mg(s) + 2 HCl(aq) MgCl(aq) + H(g)
50.0 mL of 3.0 M HCl reacts with excess Mg in a closed 4.0 L container at 295 K. What is the pressure from the hydrogen gas that is produced?