Combined Gas Law
What will be the final pressure of a sample of nitrogen with a volume of 955 mL at 745 torr and 25 C if it is heated to 62 C and given a final volume of 1155 mL?
Related Problems
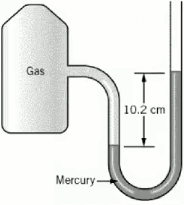
A student collected a gas in an apparatus connected to an open end manometer. The difference in heights of mercury in the two columns was 102 mm and the atmospheric pressure was measured to be 756 mmHg. What was the pressure of the gas in the apparatus in atm?
A sample of nitrogen has a volume of 883 mL and a pressure of 741 torr. What pressure will change the volume to 655 mL at the same temperature?
How many liters of hydrogen, measured at STP, are needed to combine exactly with 1.50 L of nitrogen, also at STP, to form ammonia?
n + 3 H 2 NH
How many grams of Ar are in a 12.0 L cylinder of argon at a pressure of 57.8 atm and a temperature of C ?