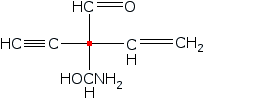
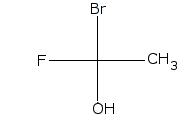
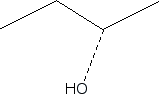
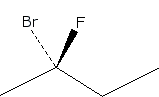
S and R configurations
Is the stereocenter labeled red in the S or R configuration?
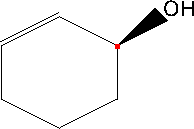
To determine whether the stereocenter labeled in red has an S or R configuration, you will first need to apply the Cahn-Ingold-Prelog priority rules to assign priorities to the substituents attached to that carbon. The substituent with the highest atomic number will be assigned the highest priority, and this process continues until all groups are ranked.
Once the priorities are established, visualize the molecule in three dimensions, noting that the thick line indicates the substituent is projecting out towards the viewer. To analyze the configuration, position yourself so that the lowest priority substituent is pointing away from you. If the sequence of the remaining groups, when viewed from this orientation, follows a clockwise direction, the configuration is classified as R. Conversely, if the sequence appears counterclockwise, the configuration is classified as S. This process is crucial for understanding the stereochemistry of the molecule, as it can significantly influence its chemical properties and reactivity in various reactions.
Related Problems
Assign priorities for the substituents attached to the stereocenter labeled red.