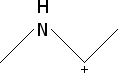
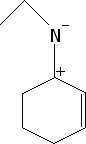
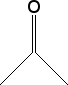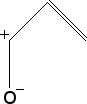Cycloalkane Resonance Structures
Draw the resonance structure(s) for
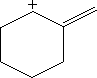
In this problem, you're asked to draw the resonance structures for a cycloalkane with a positive charge on one carbon and a double bond on the adjacent carbon. The key idea here is that the electrons from the double bond can be "donated" or shifted toward the positively charged carbon. This creates a situation where the positive charge is not fixed in one place but can move around, meaning it's distributed across different carbons in the ring.
Resonance structures allow us to represent this movement of electrons by showing multiple valid configurations of the molecule. In each resonance structure, the position of the double bond and the positive charge will shift, but the overall structure of the cycloalkane remains the same. This delocalization of charge makes the molecule more stable than if the charge were localized on a single carbon atom.